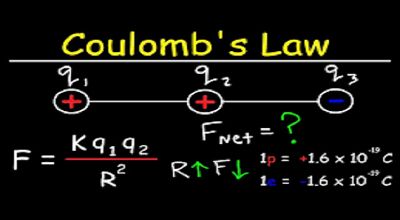
Coulomb’s Law
Coulomb’s Law is a fundamental principle in electromagnetism that describes the electrostatic force between charged particles. This force governs the interactions between electrically charged objects and is crucial in understanding various phenomena in physics and engineering.
Historical Context and Development
Coulomb’s Law is named after Charles-Augustin de Coulomb. A French physicist who first published the law in 1785. His experiments with charged objects laid the groundwork for understanding the quantitative nature of electrostatic forces.
Mathematical Formulation
Coulomb’s Law is mathematically expressed as:
F=kq1q2r2F = k \frac{q_1 q_2}{r^2}F=kr2q1q2
where:
- FFF is the electrostatic force between two point charges q1q_1q1 and q2q_2q2,
- rrr is the distance between the charges,
- kkk is Coulomb’s constant, a proportionality constant in the vacuum (k≈8.99×109 N m2/C2k \approx 8.99 \times 10^9 \, \text{N m}^2/\text{C}^2k≈8.99×109N m2/C2).
Key Concepts and Definitions
Point Charges
- Point charges are idealized objects with negligible size compared to the distance between them.
Electrostatic Force
- The electrostatic force is the force exerted between charged objects due to their electric fields.
Coulomb’s Constant
- Coulomb’s constant, kkk, determines the strength of the electrostatic force in a vacuum.
Conditions for Validity
Coulomb’s Law applies under certain conditions:
- Charges must be stationary (not accelerating).
- Charges must be point-like or distributed such that they can be treated as point charges.
- The medium between charges should be a vacuum or a non-conductive material to avoid significant polarization effects.
Application of Coulomb’s Law
Force Calculation
Coulomb’s Law allows us to calculate the force between two point charges. For example, consider two charges q1q_1q1 and q2q_2q2 separated by a distance rrr:
F=kq1q2r2F = k \frac{q_1 q_2}{r^2}F=kr2q1q2
Direction of the Force
The force is attractive if the charges are of opposite sign and repulsive if they are of the same sign.
Superposition Principle
Coulomb’s Law follows the superposition principle, meaning that. The total force on a charge due to multiple other charges is the vector sum of the forces exerted by each charge.
Practical Examples
Point Charge Interaction
- Calculating the force between electrons in an atom.
- Understanding the forces between ions in a crystal lattice.
Continuous Charge Distributions
- Approximating the force between charged plates with uniform surface charge densities.
Forces in Electric Fields
- Describing the interaction of charged particles in an electric field.
Limitations and Extensions
Non-Point Charges
- Coulomb’s Law can be extended to deal with extended charge distributions through integration.
Dielectric Materials
- In materials other than vacuum, the electrostatic force is reduced by a factor called the dielectric constant.
Experimental Verification
Cavendish Experiment
- The Cavendish experiment in the late 18th century verified the inverse-square nature of the electrostatic force.
Modern Experiments
- High-precision measurements continue to confirm Coulomb’s Law in various contexts, including atomic and subatomic scales.
Conclusion
Coulomb’s Law remains a cornerstone in electromagnetism, providing a quantitative description of the electrostatic force between charged particles. Its applications span from atomic interactions to macroscopic phenomena. Underpinning much of our understanding of electricity and magnetism. As our knowledge of physics advances. The Coulomb Law continues to serve as a robust framework for exploring new frontiers in science and technology.